CTC Story
Cancer & CTC History
 >
CTC Story
>
Cancer&CTC History
>
CTC Story
>
Cancer&CTC History
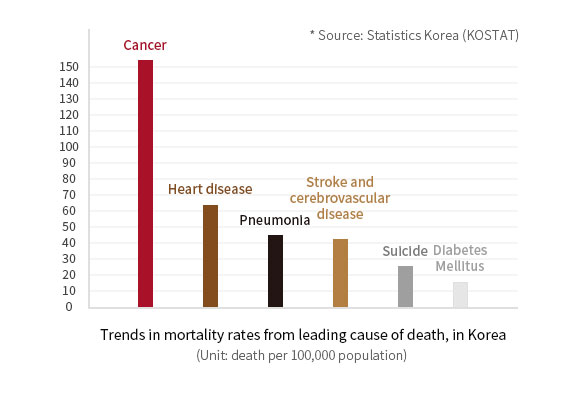
Cancer
Masses of uncontrolled rapidly grown cells in the body become cancerous and some of them develop as malignant tumors prone to penetrate and spread other tissues.
Cancer is the first leading cause of death in Korea and is the greatest life threat to human life.
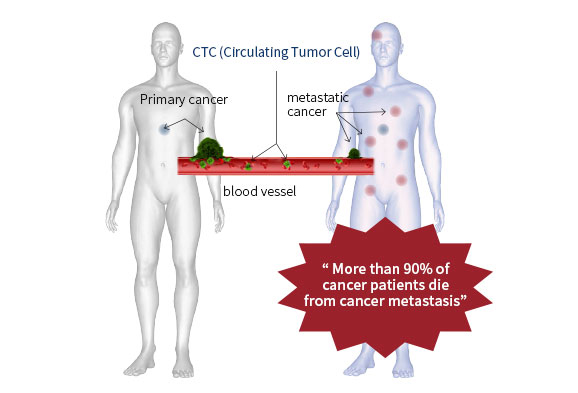
CTC
Circulating Tumor Cells (CTCs) are shed from primary sites of cancer and circulates along the blood vessels. Cancer metastasis cause 90 percentage of cancer deaths and CTCs may represent initiating steps of metastasis. Thus, understanding the roles of CTCs in cancers will pave the new way in the development of future cancer therapies.
-
1850
- Surgical removal
- Tumor removal by surgery was possible due to the introduction of anesthesia (1846) and disinfectant (1865) but not applicable to metastatic cancer.
- Discovery of CTCs (1869)
- Professor Ashworth discovered a distinctive cell type from the blood of a deceased with cancer metastasis that had similar morphology with cancer cells. Radiation therapy
-
1900
- Radiation therapy
- Emil Grove, a medical student at the University of Chicago, proposed the application of powerful X-rays for cancer treatment (1896). It worked effectively in a limited area of cancer, not as much as in metastatic sites. It was also known to cause adverse DNA mutations in healthy surrounding tissues.
- Seed and Soil hypothesis (1889)
- Professor Paget claimed that CTCs were the main cause of cancer metastasis in seed and soil theories.
-
1950
- First chemotherapy
- Dr. Sydney Farber used aminopterin, a folate antagonist, to a pediatric leukemia patient as the first chemotherapy in 1948.
- Researches of CTCs in continuation
- Several researchers had continued to identify the relevance between CTC and cancer metastasis.
-
1970
- Chemotherapy (1st generation)
- In 1955, the Cancer Chemotherapy National Service Center was founded in the US. These chemicals induced cell death in rapidly proliferating cells less effective in cells with normal proliferation hence removed cancerous cells. However, they brought on a significant adverse effect that kills normal cells.
- Identification of CTC's Survival in blood (1970)
- CTCs lasted long in harsh blood environments (Fidler, J Natl Cancer Inst)
- Advances in Molecular Biology Research
- Molecular identification of oncogenes and tumor suppressor genes led landmark breakthroughs in anti-cancer therapeutics.
-
2000
- Targeted Therapy (2nd Generation)
- Cancer cells express distinctive biomarkers that make cancers grow and spread cancers. Targeted therapy focuses on attacking those biomarkers to hinder downstream signaling pathways, consequently reduce and block cancer dissemination. Although the 2nd generation therapy was significantly reduced adverse effects compared to 1st generation chemo-drugs, its application arose another problem i.e., resistance against the drug as a result of subsequent genetic mutations in cancers.
- CTC separation technology approved by FDA (2004)
- The FDA has approved epCAM-based CTC separation technology.
- Accumulation of CTC clinical data
- There have been accumulating reports showing the relevance of CTCs to cancer metastasis.
-
2010
- Immunotherapy (3rd generation, 2010)
- mmunotherapies for cancer use the body’s natural defense system to fight cancer.
- This treatment stimulates direct or indirect immune responses to cancer.
- Development of CTC Separation Technology
- Various methods have been developed to isolate CTCs from blood using ligand interaction with CTCs or physical properties of CTCs.
- CytoGen’s technology in CTC isolation
- CytoGen Inc. was established with the confidence of advanced technology in isolation of CTC from blood using High-Density Micro-porous (HDM) chips and gravity-based filtration method.
- CytoGen’s CTC-based Liquid Biopsy Platform (2016)
- CytoGen completed the development of CTC based liquid biopsy platform. It is a full automation liquid biopsy platform, which supports separation, immuno-fluorescent staining, and analysis of CTCs hence minimizes errors in CTC characterization.



